Introduction
Staff
Vivienne Thompson Vivienne.Thompson@icr.ac.uk and Sara Kisakye-Nambozo Sara.Kisakye-Nambozo@icr.ac.uk
Purpose
The baculovirus (BV) expression service will routinely prepare viruses from recombinant BAC-TO-BAC plasmids for staff within the section. Users can book the expression of recombinant proteins in BV infected cells.
General Introduction to BV Expression
BV expression is suitable for the high level expression of recombinant proteins in a eukaryotic cell environment. BV expressed proteins are generally soluble, and are post translationally modified (glycosylated, fatty acid acylated phosphorylated and methylated). Yields of up to 100 mg/L have been reported. The BV system is particularly well suited to the expression of multiple proteins using a dual BV expression vector or co-infection.
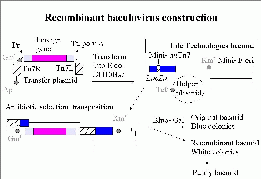
Preparation of Recombinant BVs
Recombinant baculoviruses are prepared in our labs using the Life Technologies Bac-to-Bac system. Life Technologies have created a bacmid which is a 130 kb plasmid containing BV genes, a kanamycin resistance gene and an E.coli origin of replication. The Invitrogen bacmid has been improved by Imre Berger and colleagues (a report is available in Nature) by deletion of the v-cath protease and chiA chitinase genes to prevent degradation during BV expression.
click image for larger version
It also contains a Tn7 attachment site engineered within a lacZ alpha peptide sequence, such that it doesn't disrupt the coding sequence. The gene of interest is first cloned into a Bac-to-Bac plasmid, flanked by Tn7 sequences. This is then transformed into the E.coli DH10BAC which contains the bacmid, and a helper plasmid encoding the transposase. The foreign gene is transposed on to the bacmid and selected by growth in the presence of gentamicin, kanamycin and tetracycline. The original bacmid encodes the lacZ alpha peptide which complements with the chromosomal beta peptide to form a fully functional beta galactosidase which cleaves bluogal and produces blue colonies. The transposed foreign gene disrupts the lacZ alpha peptide, and thus recombinant bacmid clones are white.
Bacmid DNA is prepared from the putative recombinant clones, and the transposition is confirmed by PCR. The recombinant bacmid is transfected into Sf9 cells, and 3 days later infective virus can be harvested from the surrounding medium. Subsequent rounds of viral amplification evenutally yield a high titer stock which can be used to infect Sf9 cells for protein expression.
click image for larger version
Expression variables
Expression is usually optimal after a 3 day infection. We have not seen any dependance on M.O.I., with values of 1 - 10 giving similar levels of recombinant protein. Expression of non secreted proteins in Sf9 cells is better than or the same as expression of the same protein in High Five cells.
Purification considerations
In our experience, large amount of Sf9 lysate bleach IMAC resins. This does not appear to be true of High Five lysates. The problem of insect media containing EDTA and histidine bleaching IMAC columns has been widely identified within the baculovirus expression community. Invitrogen advise that there is no EDTA in the formulation of their SF900II insect media (which we use) but there is histidine and F 68. Invitrogen reccomend that we diafiltrate the supernatant. Invitrogen technical services do not think the pluronic is responsible for the stripping of column, but the media does strip Ni columns. The pH of insect media is also a little lower than ideal for IMAC.
On a larger scale, Invitrogen suggest using dialfiltration to buffer exchange the sup prior to loading. This should remove the offending materials (but not much the F68) and can get a into a pH range better. One caution statement: do not try to raise the pH of the insect media as a ppt will form. The insect media is around 5.8-6.2. One could either diafilter/concentrate using a buffer of similar pH until the majority of components have passed through or concentrate then gradually add the column loading buffer until most of the medium compnents have passed through.
Invitrogen do not know which component(s) are responbile for the stripping. Their technical services department have thought of adding back di- and trivalent cations the medium to reduce stripping and on a very small scale had some positive effects, but it could make process development and potentially waste disposal more complex
From the formulation of the medium, and previous experience, Invitrogen understand the material in question to be the triglycerides, sterols, phospholipids, non-ionic detergents (Tween-80) and most significantly, the block co-polymer non-ionic surfactant, Pluronic F-68, which are all standard components of the medium.
To remove these components prior to protein purification, Invitrogen recommend use one of the following columns:
Cloning considerations
Vector maps are given in Appendix A of the BAC-TO-BAC Baculovirus Expression Systems Instruction Manual, available as a PDF file in the Tech-online section of Life Technologies web page. It is best to eliminate leader (untranslated sequence) from the insert which is to be cloned into pFastBac1, and to have the ATG in the insert as close to the polyhedrin promoter as possible (ie. clone into the BamH1 site). The sequence in pFastBac1 between the polyhedrin promoter and the BamH1 site contains the naturally 5' untranslated region of the polyhedrin gene, as well as the elements that help to confer good translation. It may be advantageous to delete the sequence between the ATT (original translational start) and the BamHI site according to some reports in the literature. In the polyhedrin promoter region, the start of transcription is the TAAG at 3945. The original ATG (now an ATA) is at 3994. From the ATA to the first restriction enzyme site can definitely be eliminated. None of the 5' UTR can be eliminated. In the original vectors, Life Technologies tried to eliminate the 5' UTR and saw almost no expression. The pFastBac dual vector allows one gene to be expressed under the control of the p10 promoter and one gene to be expressed under the control of the polyhedron promoter. p10 is activated slightly ahead of the polyhedron promoter and the expression from the p10 promoter is slightly lower than the polyhedron promoter.
Some additional vectors have been created by members of the lab at the ICR and by colleagues at ETH Zürich (see the report in Nature).
Modified
Bac-to-Bac Plasmid Transfer Vectors available at the
ICR |
||
| Vectors | Features | Origin |
| pFastbacFLAG | N-term FLAG tag (cut by PreSc) | Laura Pickles |
| pFastbacNTAP | N-term TAP tag | Lori Passmore |
| pFastBac1-CBP | C-term CBP tag (cut by PreSc) | James Parker |
| pTWO-B | N-term HIS tag (cut by PreSc) | Tony Oliver |
| pFastbacGST | N-term GST tag (cut by PreSc) | Paul Wan |
| pFBDM | pFastbac DUAL derivative engineered to allow iterative cloning | Imre Berger and colleagues |
Resolving confusion between pFastBacDUAL and pFBDM vector diagrams
The Invitrogen Bac-to-Bac manual describes the pFastBacDUAL polyhedron promoter multiple cloning site as 'I' and the p10 promoter multiple cloning site as 'II', where as Berger et al. name the multiple cloning sites in pFBDM the other way round. In addition, Berger et al. use isoschizomers for the names of the restriction sites, rather than using the names given in the Bac-to-Bac manual. To summarise, the multiple cloning sites are as follows.
Polyhedron promoter (MCSI in pFastBacDUAL, MCSII in pFBDM)
p10 promoter (MCSII in pFastBacDUAL, MCSI in pFBDM)
pFBDM contains a multiplication module which allows iterative cloning of the genes in MCSI and II. It can be used to combine genes for multiple interacting proteins, or increase expression from one gene by increasing copy number. The non multiple cloning sites for PmeI, AvrII, BstZ171 and SpeI are used for the iterative cloning, so the SpeI site in MCSII must be eliminated during cloning.
Alternative BV systems
Experience within the ICR has shown the Invitrogen Bac-to-Bac system to be capable of generating significant quantities of recombinant protein from the majority of genes tested. However, there are some circumstances where an alternative approach may be justified.
The pIEX and pBiEx vectors are designed to allow rapid protein expression in insect cells without the time-consuming process of creating a recombinant baculovirus. The plasmids are transiently transfected into insect cells using Insect Gene Juice, and then direct expression of the recombinant gene using the the vector encoded hr5 enhancer and the IE1 (immediate early) promoter. The pIEX vectors can also be otained as prepared Ek/LIC vectors which allow rapid directional cloning of PCR-amplified DNA. Using specifically designed primers for amplification, inserts can be efficiently cloned without the need for restriction enzyme digestion or ligation. The pBiEx vectors are used for expression in insect cells and for IPTG inducible expression in E. coli (T7lac promoter). The pIEX and pBiEx vectors has similar (but not identical) multiple cloning sites and a variety of tags. Unfortunately, these vectors do not have 3 versions which would allow easy in frame cloning, so unless you clone using PCR or your current restriction sites happen to be suitable, you may consider the cloning too complex to be bothered with these vectors. The pIEX and pBiEx N-terminal vector encoded tag sequences can be removed by cloning into the PshA I or BglII sites, respectively, then cleaving the fusion protein with enterokinase. The transient expression of plasmids in insect cells is only useful for the generation of small amounts of protein. However, it can be used to screen a large number of constructs to identify to identify a stable, well expressed, soluble domain. Once this domain has been identified, the relevant portion of the gene would then be recloned into a Bac-to-Bac plasmid, for the preparation of a suitable baculovirus for high level expression.
The flashBAC system allows the production of pure recombinant baculovirus clones without the time consuming E. coli transposition stages required for the Bac-to-Bac system. The disadvantage of the flashBAC system is that it is expensive, but its use may be justified on the rare occasions when the Bac-to-Bac transpositon fails to produce a recombinant bacmid. We are currently investigating use of the flashBAC system with pBac PAK8 Vector.